

This nonmetallic element is the key to the chemistry of living organisms, primarily due to its tetravalent state, which allows it to form four covalent chemical bonds with other atoms. It is typically located as a subscript to the left of the element symbol. Carbon is the element with atomic number 6 on the periodic table with symbol C. The element carbon (C) has an atomic number of 6, which means that all neutral carbon atoms contain 6 protons and 6 electrons. Atomic numbers and mass numbers are always whole numbers as they are obtained by counting protons, neutrons, and electrons. The mass number is determined by the number of protons and neutrons combined. The atomic number is the smaller of the two numbers in the symbol. The number of protons that a chemical element has in its centre (nucleus) is called the atomic number. For example, if the symbol is written as 14 6C, the number "6" is listed. More commonly, the isotope symbol already tells you the atomic number.For example, if the symbol is 14C, you know the element symbol is C or that the element is carbon. You can use the symbol to look up the number. There is more than one way to write an isotope symbol, but the element symbol will always be included. You can find the atomic number from an isotope symbol.Calculate the number of protons, neutrons and electrons it contains. For example, if you are told the element name is aluminum, you can find the name or symbol Al to determine the atomic number is 13. The atomic number of a sodium atom is 11 and its mass number is 23. While other numbers may be decimal values, the atomic number is always a simple positive whole number. There may be many numbers on a periodic table, so how do you know which one to pick? The atomic numbers go in order on the table. Symbol: C Atomic Number: 6 Atomic Mass: 12.0107 amu Melting Point: 3500.0 ☌ (3773.15 K, 6332.0 ☏) Boiling Point: 4827.0 ☌ (5100.15 K, 8720. In a typical sample of carbon-containing material, 98.89 of the carbon atoms also contain 6 neutrons, so each has a mass number of 12. Atoms of the same element with different mass numbers are called isotopes. The element carbon (C) has an atomic number of 6, which means that all neutral carbon atoms contain 6 protons and 6 electrons. The number of protons and the mass number of an atom define the type of atom. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. About Transcript Each atom has a charged sub-structure consisting of a nucleus, which is made of protons and neutrons, surrounded by electrons. The nucleus is composed of protons and neutrons.
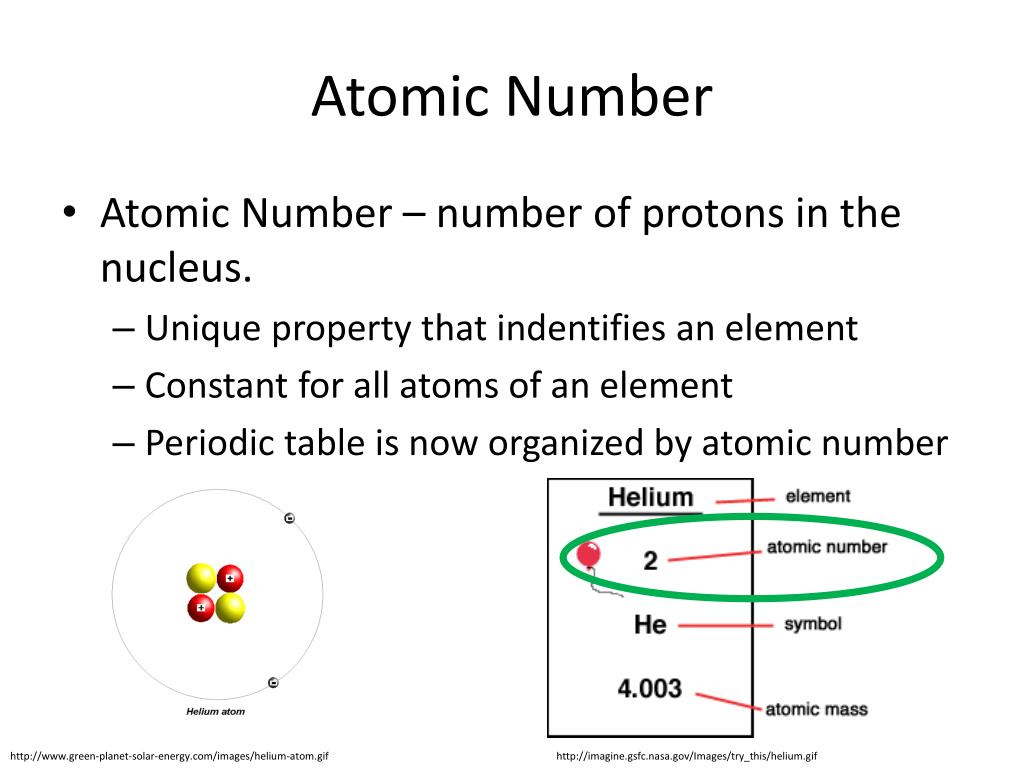
If you have an element name or symbol, use a periodic table to find the atomic number. The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons.This must include Group of answer choices a emission an emission a emission a & emission. The classification of elements by atomic number allows us to understand many properties of the atom and makes it possible to predict behaviors instead of just having to memorize everything. An element of atomic number 83 in a relatively higher energy state decays radioactively to an element of atomic number 84 with lower energy level. (Credit: User:Cepheus/Wikimedia Commons Source: (opens in new window) License: Public Domain) \): The periodic table classifies elements by atomic number.


 0 kommentar(er)
0 kommentar(er)
